Abstract
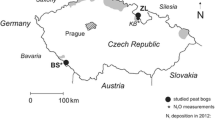
Sulfur transformations in freshwater peat were studied using contrasting stable isotope signatures of atmospheric input (high δ34S) and Sphagnum peat substrate (low δ34S). Wet subsurface peat samples from the Lehstenbach watershed, Fichtelgebirge, Germany were incubated anaerobically at 5 and 15°C. Pore-water sulfate was augmented with natural precipitation at the onset of the experiments. Sulfate concentrations and δ34S ratios of residual pore water were measured in 1-day intervals (9 days) and 1-week intervals (7 weeks) at 15°C, and in 1-week intervals (7 weeks) at 5°C. Initially, SO4 2− concentrations decreased (by 50 to 85%) and δ34S ratios increased (by as much as 16‰) at both temperatures due to bacterially-mediated dissimilatory sulfate reduction. At the higher temperature (15°C), the S isotope effect (Δ δ34S) was higher than at the lower temperature (5°C). On day 4 (at 15°C) and day 29 (at 5°C), the δ34S ratio of pore-water sulfate started to decrease by as much as 20‰ The changing S isotope composition provided evidence for a dynamic turnover of the pore-water sulfate pool in anaerobic peat. The observed δ34S pattern could not be explained solely by isotope selectivity of the sulfate-reducing bacteria. Sulfur isotope data indicated a replenishment of the sulfate pool by hydrolysis of ester-sulfate.
Similar content being viewed by others
Literature cited
Alewell, Ch. and M. Gehre. 1999. Patterns of stable S isotopes in a forested catchment as indicators for biological turnover. Biogeochemistry 47:317–331.
Alewell, Ch. and A. Giesemann. 1996. Sulfate reduction in a forested catchment as indicated by δ34S values of sulfate in soil solutions and runoff. Isotopes in Environmental and Health Studies 32:203–210.
Altschuler, Z. S., M. M. Schnepfe, C. C. Silber, and F. O. Simon. 1983. Sulfur diagenesis in Everglades peat and origin of pyrite in coal. Science 221:221–227.
Bloomfield, C. and J. K. Coulter. 1973. Genesis and management of acid sulphate soils. Advances in Agronomy 25:265–326.
Brown, K. A.. 1985a. Formation of organic sulphur in anaerobic peat. Soil Biology & Biochemistry 18:131–140.
Brown, K. A.. 1985b. Sulphur distribution and metabolism in waterlogged peat. Soil Biology & Biochemistry 17:39–45.
Brown, K. A. and J. F. Macqueen. 1985. Sulphate uptake from surface water by peat. Soil Biology & Biochemistry 17:411–420.
Chae, Y. M. and H. R. Krouse. 1986. Alteration of sulfur-34 natural abundance in soil by application of feedlot manure. Soil Science Society of America Journal 50:1425–1431.
Clymo, R. S.. 1984. The limits to peat bog growth. Philosophical Transactions of the Royal Society London, B Biological Sciences 303:605–654.
David, M. B., S. C. Schindler, M. J. Mitchell, and J. E. Strick. 1983. Importance of organic and inorganic sulfur to mineralization processes in a forest soil. Soil Biology & Biochemistry 15:671–677.
Faure, G.. 1998. Principles and Applications of Geochemistry. Prentice Hall. Upper Saddle River, NJ, USA.
Giblin, A. E. and R. K. Wieder. 1992. Sulphur cycling in marine and freshwater wetlands. p. 85–124. In R. W. Howarth, J. W. B. Stewart, and M. V. Ivanov (eds.) Sulphur Cycling on the Continents. SCOPE 48. John Wiley & Sons, New York, NY, USA.
Groscheová, H., M. Novák, M. Havel, and J. Ĉerný. 1998. Effect of altitude and tree species on δ34S of deposited sulfur. Water, Air & Soil Pollution 105:295–303.
Harrison, A. G. and H. G. Thode. 1958. Mechanism of the bacterial reduction of sulphate from isotope fractionation studies. Transactions of the Faraday Society 54:84–92.
Houghton, C. and F. A. Rose. 1976. Liberation of sulfate from sulfate esters by soils. Applied and Environmental Microbiology 31: 969–976.
Howarth, R. W., J. W. B. Stewart, and M. V. Ivanov. 1992. Sulphur Cycling on the Continents. SCOPE 48. John Wiley & Sons Chichester, UK.
Jarvis, B. W., G. E. Lang, and R. K. Wieder. 1987. Arylsulphatase activity in peat exposed to acid precipitation. Soil Biology & Biochemistry 19:107–109.
Kaplan, I. R. and S. C. Rittenberg. 1964. Microbiological fractionation of sulfur isotopes. Journal of General Microbiology 26:127–163.
Krouse, H. R. and V. A. Grinenko. 1991. Stable Isotopes. Natural and Anthropogenic Sulpur in the Environment. SCOPE 43. John Wiley & Sons, Chichester, UK.
Krouse, R. H., B. Mayer, and J. J. Schoenau. 1996. Applications of stable isotope techniques to soil sulfur cycling. p. 247–284. In T. W. Boutton and S. Yamasaki (eds.) Mass Spectrometry of Soils. Marcel Dekker, New York, NY, USA.
Lamers, L. P. M., S. M. E. Van Roozendaal, and J. G. M. Roelofs 1998. Acidification of freshwater wetlands: combined effects of non-airborne sulfur pollution and dessication. Water. Air & Soil Pollution 105:95–106.
Mayer, B., K. H. Feger, A. Giesemann, and H-J Jäger. 1995. Interpretation of sulfur cycling in two catchments in the Black Forest (Germany) using stable sulfur and oxygen isotope data. Biogeochemistry 30:321–58.
Maynard, J. B. 1983. Geochemistry of Sedimentary Ore Deposits. Springer, New York, NY, USA.
Morgan, M. D.. 1994. Modelling excess sulfur deposition on wetland soils using stable sulfur isotopes. Water, Air & Soil Pollution 79: 299–308.
Novák, M., S. H. Bottrell, H. Groscheová, F. Buzek, and J. Černý. 1995. Sulphur isotope characteristics of two North Bohemian forest catchments. Water, Air & Soil Pollution 85:1641–1646.
Novák, M., F. Buzek, and M. Adamová. 1999. Vertical trends in δ13C, δ15N and δ34S ratios in bulk Sphagnum peat. Soil Biology & Biochemistry 31:1343–1346.
Novák M., J. W. Kirchner, H. Groscheová, M. Havel, J. Černý, R. Krejčí, and F. Buzek. 2000. Sulfur isotope dynamics in two Central European watersheds affected by high atmospheric deposition of SOx. Geochimica et Cosmochimica Acta (in print).
Novák, M. and R. K. Wieder. 1992. Inorganic and organic sulfur profiles in nine Sphagnum peat bogs in the United States and Czechoslovakia. Water, Air & Soil Pollution 65:353–369.
Novák, M., R. K. Wieder, and W. R. Schell. 1994. Sulfur during early diagenesis in Sphagnum peat: Insights from δ34S ratio profiles in 210Pb-dated peat cores. Limnology and Oceanography 39:1172–1185.
Schoenau, J. J. and J. R. Bettany. 1989. 34S natural abundance variations in prairie and boreal forest soils. Journal of Soil Science 40:397–414.
Spratt, H. G. Jr., M. Morgan, and R. E. Good. 1987. Sulfate reduction in peat from a New Jersey Pinelands Cedar swamp. Applied and Environmental Microbiology 53:1406–1411.
Stam, A. C., M. J. Mitchell, H. R. Krouse, and J. S. Kahl. 1992. Stable sulfur isotopes of sulfate in precipitation and stream solutions in a northern hardwood watershed. Water Resources Research 28:231–236.
Van Stempvoort, D. R., P. Fritz, and E. J. Reardon. 1992. Sulfate dynamics in upland forest soils, central and southern Ontario, Canada: Stable isotope evidence. Applied Geochemistry 7:159–175.
Wieder, R. K. and G. E. Lang. 1988. Cycling of inorganic and organic sulfur in peat from Big Run Bog, West Virginia. Biogeochemistry 5:221–242.
Wieder, R. K. and M. Novák. 1995. Biogeochemical processes during the treatment of acid mine drainage: the Kentucky wetland project. In J. Pašava, B. Kříbek and K. Žák (eds.) Mineral Deposits: from Their Origin to Their Environmental Impact. Balkema, Rotterdam, The Netherlands.
Wieder, R. K., J. B. Yavitt, and G. E. Lang. 1990. Methane production and sulfate reduction in two Appalachian peatlands. Biogeochemistry 10:81–104.
Yanagisawa, F. and H. Sakai. 1983. Precipitation of SO2 for sulphur isotope ratio measurements by the thermal decomposition of BaSO4 − V2O4 − SiO2 mixtures. Analytical Chemistry 55:985–987.
Zhang, Y., M. J. Mitchell, M. Christ, G. E. Likens, and H. R. Krouse. 1998. Stable sulfur isotopic biogeochemistry of the Hubbard Brook Experimental Forest, New Hampshire. Biogeochemistry 41:259–275.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Groscheová, H., Novák, M. & Alewell, C. Changes in the δ34S ratio of pore-water sulfate in incubated Sphagnum peat. Wetlands 20, 62–69 (2000). https://doi.org/10.1672/0277-5212(2000)020[0062:CITSRO]2.0.CO;2
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1672/0277-5212(2000)020[0062:CITSRO]2.0.CO;2